MRI-Compatible Implantable Cardioverter Defibrillators (ICDs)
Evera MRI SureScan ICD System
MANUFACTURER: Medtronic
FDA APPROVED: September 2015
FIRST APPEARANCE IN CHATTANOOGA: October 2015
NOTES: With the newest generation of ICDs, patients can undergo an MRI without complications – eliminating any need to choose between diagnostic imaging and treating a dangerous heart rhythm.
Why You Might Need an ICD
ICDs treat certain types of arrhythmia, which is any irregularity in your heart’s rhythm. You may need an ICD if you have a dangerously fast heartbeat (ventricular tachycardia), a chaotic heartbeat that makes it so your heart can’t supply enough blood to the rest of your body (ventricular fibrillation), or if you have a known risk of sudden cardiac arrest.
How ICDS Work
If you have a life-threatening arrhythmia or a known risk of cardiac arrest, a cardiologist will implant a battery-powered ICD beneath your skin under your collarbone. Two leads, or wires with electrodes, will stretch from the ICD to the heart to keep track of your heart rate. If the device detects an abnormal rhythm, it will send small electrical pulses to your heart to restore a normal rhythm. If these don’t work, it will send a low energy shock followed by a high energy shock.
Former ICDS

An Expert Weighs In
Until now if you needed an MRI but had an ICD, you were out of luck. The MRI would heat up the ICD’s wires, causing the wires to also heat up the heart muscle. Many patients needing an ICD have even forfeited the opportunity to implant one in order to continue imaging for a different condition, such as a back or joint condition. The incompatibility of ICDs and MRI also presented an obstacle to physicians looking to quickly and efficiently diagnose a stroke.
How The Evera ICD Works
The Evera MRI SureScan ICD System allows patients to safely undergo full-body MRIs, thanks to several design and material modifications. The new ICD will not overheat in response to the MRI environment, and its hardware components ensure high energy fields will not damage circuits.
Bioabsorbable Stents
SYNERGYBioabsorbable Polymer Drug-Eluting Stent System
MANUFACTURER: Boston Scientific
FDA APPROVED: October 2015
FIRST APPEARANCE IN CHATTANOOGA: November 2015
NOTES: The newest generation of stents promises a faster recovery time and lower risk of complications. Its defining feature? An absorbable polymer coating.
Why You Might Need a Stent
Stents are used to treat coronary artery disease (CAD), the most common kind of heart disease and the number one cause of death for both men and women in the U.S. In CAD, the coronary arteries become clogged with waxy cholesterol deposits called plaque. As plaque continues to build up, the arteries delivering blood to the heart muscle harden and narrow. Eventually, this narrowing can lead to chest pain (angina) and heart attack.
How Stents Work


An Expert Weighs In
Stents are a standard treatment for heart attack in progress or unstable angina, a serious form of chest pain requiring emergency treatment. During a procedure called an angioplasty, a cardiologist uses a catheter to insert a stent into the clogged artery. The stent is then left in the artery permanently to hold it open and improve blood flow.
Former Stents
Since the early 2000s, cardiologists have used drug-eluting stents, which release drugs to prevent re-narrowing of the artery. Drug-eluting stents are significantly better than their predecessors, and yet a polymer coating remains on the stent after the drug is delivered. Long-term exposure to this polymer can cause inflammation, which delays healing and has been associated with the formation of blood clots and the reappearance of plaque.
How SYNERGY Works
Unlike standard drug-eluting stents, Boston Scientific’s SYNERGY Bioabsorbable Polymer Drug-Eluting Stent System (BP-DES) has a bioabsorbable polymer coating. This means that after the drugs are fully released in the arteries – which takes three months – the stent’s polymer coating is also absorbed by the body, leaving only the stent itself behind.
Transcatheter Aortic Valve Replacement (TAVR)
Edwards's SAPIEN and Medtronic's CoreValve TAVR Platforms
MANUFACTURERS: Edwards Lifesciences and Medtronic
FDA APPROVED: November 2011 (SAPIEN)
FIRST APPEARANCE IN CHATTANOOGA: December 2011
NOTES: Five years ago if you needed a new heart valve but were ineligible for open heart surgery, you were out of options. This is no longer true due to the development of TAVR.
Why You Might Need Valve Replacement
TAVR is used to treat patients with aortic stenosis, one of the most common and serious types of heart valve disease. It occurs when the aortic valve in the heart doesn’t open all the way, preventing blood from flowing properly. In the U.S., aortic stenosis is mainly a disease of older people – a result of scarring and the buildup of calcium in the valve.
How Valve Replacement Works
If someone has severe aortic stenosis, the only effective treatment is to replace the diseased aortic valve. However, traditional aortic valve replacement surgery is an open heart surgery. First, the sternum is split down the middle, providing direct access to the heart.Then the damaged valve is removed and replaced with a mechanical valve or a biological valve made from animal or human tissue.
Traditional Valve Replacement
Valve replacement surgery has historically been an extremely invasive procedure. For those older than 70 or patients with other health conditions, the risks of open heart surgery often outweigh the benefits. Unfortunately, most people with severe aortic valve stenosis are older than 65. And research shows that without a valve replacement, half of patients with severe aortic stenosis will not survive more than an average of two years.
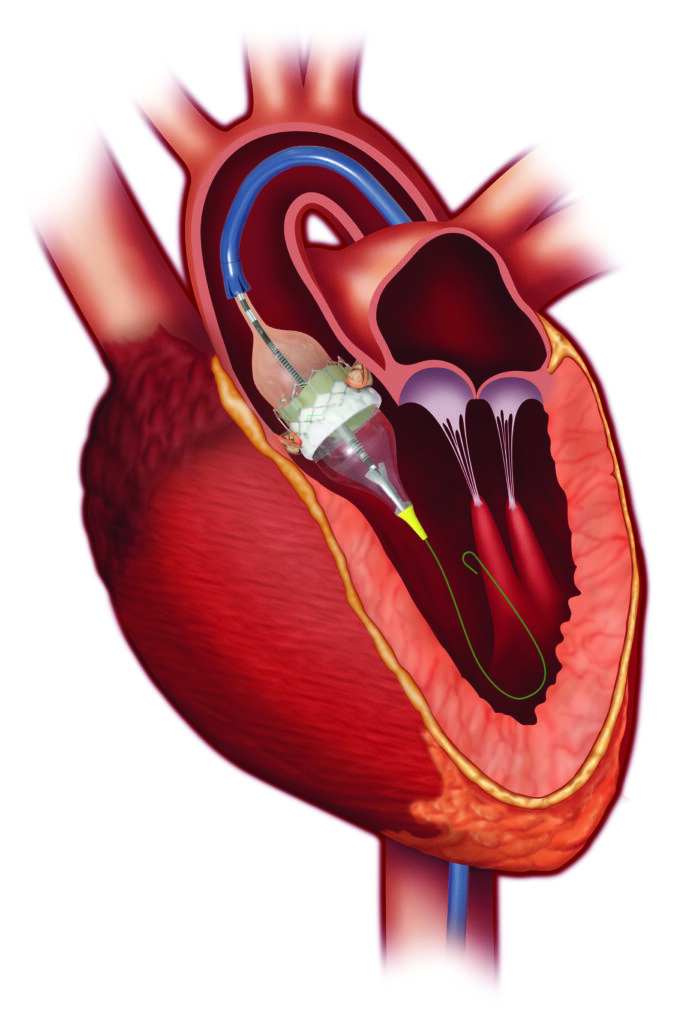

An Expert Weighs In
How TAVR Works TAVR


It is allowing doctors to perform valve replacement surgery without opening the sternum and stopping the heart. During the procedure, a surgeon makes a small incision in the leg or the chest and inserts a catheter – with the replacement valve mounted on top – into the artery. The surgeon then guides this catheter all the way to the diseased heart valve. Once in place, the replacement valve takes on the role of regulating blood flow in place of the old valve.




